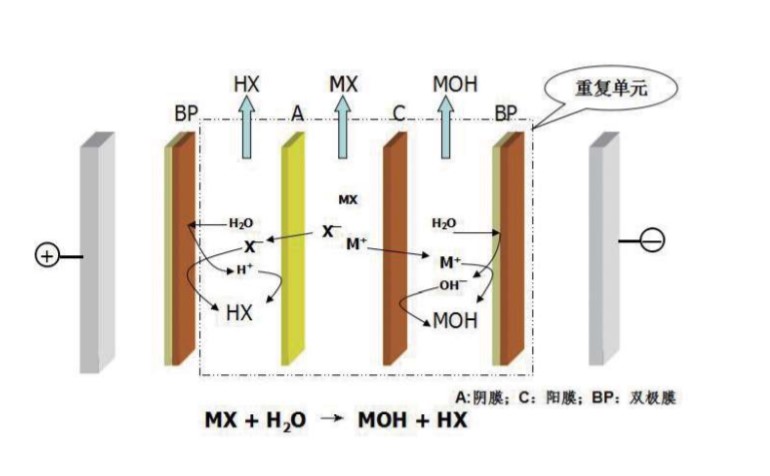
A: anion exchange membrane
C: cation exchange membrane
BP: bipolar ion exchange membrane
The principle of bipolar membrane electrodialysis
By using the potential difference as the driving force, the selective permeability of anion and cation exchange membranes, and the role of bipolar membranes to decompose water to produce H + and OH- to prepare acid and alkali separation operations.The water is catalytically active to force dissociation of water into OH- and H+ – ions when exceeding a potential difference of approximately 0.8 V In the intermediate layer between Anion Exchange Layer (AEM) and Cation Exchange Layer (CEM). The membrane should be operated under forward bias conditions which may cause blistering. The CEM must be directed towards the cathode, the AEM must be directed towards the anode. If the membrane is used in the opposite position at high current density even for short term, the interim layer may degrade (blistering), and the monolayers may delaminate.
The electro-catalytically forced water dissociation produces – in contrast to the classical electrolysis of water – no reaction gases. Therefore, one Mol of OH- and H+ – ions can be achieved at an energy value of approximately 22 Wh (Electrolysis: approximately 55 Wh per Mol).
Properties of bipolar ion exchange membranes :
Physical Properties |
Units |
Index Values |
Thickness |
mm |
0.14—0.25 |
Water content |
% |
33--40 |
Ion-exchange capacity |
mol/kg |
(Cation side)0.7-1.8 |
Selective permeability |
% |
90-95 |
Breaking Strength, max. |
MPa |
>0.25 |
Working Principle:
The bipolar membrane is a kind of electrically driven membrane. Its main function is to provide H+ ions and OH- ions under the electric field force. The membrane has one side of the cathode and the other side of the membrane. The middle layer of the cathode and the cathode is an aqueous layer. Under the action of external DC electric force, the H2O in the water layer is split into H+ ions and OH- ions, and migrates through the positive and negative sides of the subject solution respectively. Therefore, the role of the bipolar membrane is to provide H+ under the action of the electric field force. Ion and OH-ion sources, as shown:
The combination of bipolar membrane and cation exchange membrane

Applications:
·Treatment and recycling of salty wastewater· Direct production of hydrochloric acid and sodium hydroxide from salt (sodium chloride)
· Direct production of vitamin C from vitamin C sodium salt
· Direct production of tartaric acid from sodium tartrate
· Direct production of lactic acid from sodium lactate
· Direct production of gluconic acid from sodium gluconate
· Direct production of methanesulfonic acid from sodium methanesulfonate
· Direct separation of amino acids from fermentation broth
·Return from sodium acetate wastewater
Packing Size
400*800mm per sheet550*1100mm per sheet