
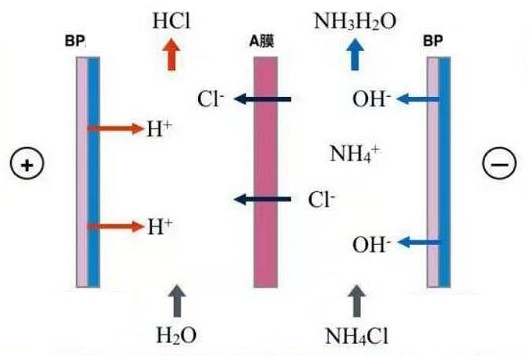
The bipolar ion exchange membrane stack is composed of a bipolar membrane, and the anion membrane and the cation membrane to form acid, alkali, and salt chambers (two chambers or three chambers) in different combinations.
The anions pass through the anion exchange membrane and combine with the H+ ions produced by the bipolar membrane to generate acid. The cations pass through the cation exchange membrane and combine with the OH-ions produced by the bipolar membrane to generate alkali. The process is equivalent to the reverse reaction process of the neutralization reaction.
The bipolar membrane electrodialysis is a new type of electrodialysis that introduces bipolar membrane on the basis of electrodialysis, and combines various configurations of cathode and/or cation membrane. Its role is to convert salt solutions into acids and bases.
Under the action of an external reverse DC electric field, the bipolar membrane dissociates H 2 O molecules into H+ and OH- under the action of a catalyst, and migrates into the solution on both sides of the membrane through the anode and cathode layers respectively. The decrease of H+ and OH concentration in the catalytic layer makes the hydrolysis reaction continue to proceed. The bipolar membrane is equivalent to a hydrolysis generator that produces H+ and OH ions.
Bipolar membrane electrodialysis is a new type of electrodialysis formed by introducing a bipolar membrane on the basis of electrodialysis and combining various configurations of the anion membrane and/or the anode membrane. Unlike the concentrated desalination function of conventional electrodialysis, its function is to convert a salt solution into an acid and a base. Under the action of a reverse DC electric field, the H2O molecules in the catalytic layer are dissociated into H+ and OH- under the action of a catalyst, and migrated through the positive and negative layers to the host solution on both sides of the membrane, respectively. The lowering of the H+ and OH- concentrations causes the hydrolysis to proceed continuously. Simply put, a bipolar membrane is equivalent to a hydrolysis generator that produces H+ and OH-ions.
The bipolar membrane, the combination of the anion membrane and the yang membrane constitute a three-chamber of acid, alkali and salt. The anion of the salt passes through the anion membrane and combines with the H+ ion generated by the bipolar membrane to form an acid. On the other hand, the cation passes through the cation exchange membrane and the double The OH-ion generated by the polar membrane combines to form a base, and the process is equivalent to the reverse reaction process of the neutralization reaction.
Characteristics of Electrodialysis Membrane Stack
- Energy saving and environmental protection, a new generation of acid-base technology;
- splitting water into H+ and OH- at low voltage;
- The acid and alkali are formed from inorganic salts and organic acid salts in a single process, and the process is simple and clean;
- The concentration of acid and alkali produced is controllable and adjustable;
- no redox reaction occurs except for the electrodes at both ends, and no by-products are produced;
- Each chamber does not require electrodes, and the amount of gas generated is small. The investment cost and operating cost account for only 40 to 50% of the electrolysis method.
Type:
Three Chambers |
Two Chambers |
|||||
Type |
SYABCM12060 |
SYABCM8040 |
SYBCM12060 |
SYBCM8040 |
SYABM12060 |
SYABM8040 |
Membrane sheet size (mm) |
550*1100 |
400*800 |
550*1100 |
400*800 |
550*1100 |
400*800 |
Membrane sheet pairs |
30-200 |
10-200 |
30-200 |
10-200 |
30-200 |
10-200 |
Stardand pairs |
50/100/200 |
50/100/200 |
50/100/200 |
50/100/200 |
50/100/200 |
50/100/200 |
Effective area(m2) |
23/46/92 |
12.5/25/50 |
23/46/92 |
12.5/25/50 |
23/46/92 |
12.5/25/50 |
Inlet water flow(m3/h) |
2.5/5/10 |
1.5/3/6 |
2.5/5/10 |
1.5/3/6 |
2.5/5/10 |
1.5/3/6 |
Processing capacity(NaCl kg/h) |
13.5/27/54 |
7.5/15/30 |
13.5/27/54 |
7.5/15/30 |
13.5/27/54 |
7.5/15/30 |
Acid concentration (mol/L) |
≥1.5 |
≥1.5 |
≥1.5 |
≥1.5 |
≥1 |
≥1 |
Alkali concentration (mol/L) |
≥1.5 |
≥1.5 |
≥1 |
≥1 |
≥1.5 |
≥1.5 |
Optimum operating temperature (℃) |
25--35 |
25--35 |
25--35 |
25--35 |
25--35 |
25--35 |
Principle of bipolar membrane with two chambers
1, Principles of preparing acid and alkali from strong base and weak acid salt-ACM
This principle is mainly used for the preparation of organic acids such as gluconic acid, malic acid, vC, propanesulfonic acid, oxalic acid, citric acid, tartaric acid, EDTA, lactic acid, methionine, morpholine ethanesulfonic acid, etc. The bipolar membrane process has no waste water discharge and by-product hydroxide Sodium can be recycled for the fermentation process.

This principle is mainly used in the preparation of organic bases such as triethylamine hydrochloride, triethylamine tetramethylammonium salt, ornithine tetrapropyl bromide, quaternary amine base, tetraethylammonium hydroxide, methyldiethanolamine, etc. When hydroxide is introduced, cations are not added, and anions are separated.
Applications Area:
1, Preparation of acid and bases from inorganic salts (such as sodium sulfate, sodium chloride, lithium chloride, etc.)2, Production of organic acids (such as gluconic acid, lactic acid, malic acid, succinic acid, etc.)
3, Production of organic bases (such as ornithine, lysine, arginine, histidine, etc.)
Inlet water requirement of Bipolar ion exchange membrane electrodialysis:
1) water temperature : 5-40 degrees Celsius2) oxygen consumption: < 20mg/L , It cannot contain aromatic hydrocarbons
3) SS: < 1mg/L
4) Fe: < 0.3mg/L
5) Mn: < 0.1mg/L
6) SDI: < 3
7) Mg, Ca < 1mg/L
8) turbidity < 1NTU
9) SiO2 < 1mg/L
Performance Introduction of SYABCM200
200 pieces of bipolar ion exchange membrane,anion-exchange membrane and cation-exchange membrane for each.
The effective area of each membrane is 50 m2.
Current Density: 500A/m2
Under standard operating conditions, the conversion efficiency of the membrane stack is as follows(Other salts can be calculated according to the corresponding equivalent molar mass):
Name |
Reaction Formula |
Processing Capacity |
Power Consumption |
NaCl |
H2O+NaCl→HCl+NaOH |
30 |
1.5 |
Na2So4 |
Na2SO4+H2O→H2SO4+NaOH |
36.4 |
1.2 |
LiCl |
LiCl+H2O→LiOH+HCl |
21.8 |
2.1 |
Li2SO4 |
Li2SO4+H2O→H2SO4+LiOH |
28.2 |
1.6 |
NaNO3 |
NaNO3+H2O→HNO3+NaOH |
43.6 |
1.1 |